- Contact Us
-

Tel:
+86-571-86879123 -

Fax:
+86-571-86910580
Home > ABOUT US > Development History
-
2023
In 2023, the company’s new product JiTanSu (Fosaprepitant dimeglumine for injection)
-
2022
In 2022, the company’s new product JiFuWei (Fulvestrant injection) was approved for marketing.
-
 2014
2014In 2014, the company started a new Three-year Plan and entered into an accelerated developement period.
-
 In October, 2013
In October, 2013In October, 2013, the company established a development strategy of “Marketing First, Total Marketing” and the marketing work entered into a new reformed period.
-
In July, 2013
In July, 2013, Mr. Fu Hang was appointed as general manager of Jiuyuan Gene, and Jiuyuan developed into a new stage.
-
2011
In 2011, the company established an Academician Expert Working Center, and Prof. Yang Shengli, the Chairman of Biotechnologist Committee of Chinese Academy of Sciences and Academician of Chinese Academy of Engineering, acted as the Science Consultant.
-
2010
In 2010, the new product recombinant human bone repairing material BMP-2 (GUYOUDAO) was officially manufactured and launched on market.
-
 2008
2008The company was awarded as one of the first “National High-Tech Enterprises” in 2008. On December 19, the 5th product JIOUTING (Palonosetron Hydrochloride API and injection) officially got approval for manufacture.
-
 In July, 2006
In July, 2006In July, 2006, the company initiated the production and sales of the 4th product YINUOJIA (Enoxaparin Sodium Injection),indicating the first officially approved enoxaparin was born in China.
-
 On September 18, 2003
On September 18, 2003On September 18, 2003, the company achieved a New Drug Certificate and Manufacture Approval of JIJUFEN, a platelet promoting drug (Recombinant Human Interleukin 11).
-
 In January, 2002
In January, 2002In January, 2002, the company established a Post-doctoral Working Station, the first post-doctoral working station for biopharmaceutical industry in Zhejiang Province.
-
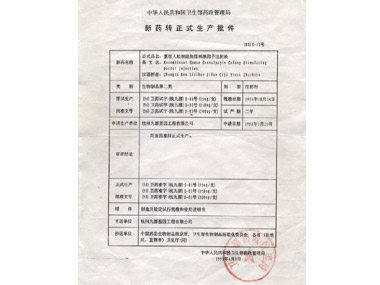 In June, 1998
In June, 1998In June, 1998, the drug low molecular weight heparin sodium injection (JIPAILIN) was approved for production and marketing by Ministry of Health in China.
-
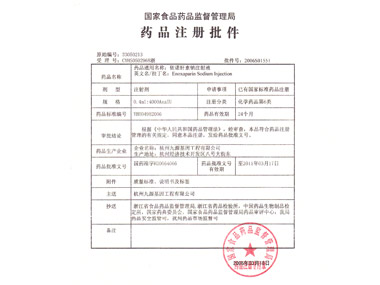 On October 14, 1996
On October 14, 1996On October 14, 1996, the company obtained the Trial-production Approval Number and New Drug Certificate of JILIFEN, the first rhG-CSF injection in China, making China become the third country able to manufacture the drug in large volumes following the U.S and Japan.
-
On December 31, 1993
Hangzhou Jiuyuan Gene Engineering Co., Ltd. was registered and established in Hangzhou New High-tech Development Zone.