- Contact Us
-

Tel:
+86-571-86879123 -

Fax:
+86-571-86910580
Home > PRODUCT CENTER > Product Introdcution
JIFUWEI(Fulvestrant Injection)
Chemical Name
Fulvestrant Injection
Specification
250mg in 5ml
Fulvestrant Injection is an estrogen receptor antagonist indicated for the
treatment of:
1. Hormone receptor (HR)-positive, human epidermal growth factor
receptor 2 (HER2)-negative advanced breast cancer in postmenopausal
women not previously treated with endocrine therapy.
2. HR-positive advanced breast cancer in postmenopausal women with
disease progression following endocrine therapy.
3. HR-positive, HER2-negative advanced or metastatic breast cancer in
postmenopausal women in combination with ribociclib, as initial
endocrine based therapy or following disease progression on endocrine
therapy.
4. HR-positive, HER2-negative advanced or metastatic breast cancer in
combination with palbociclib or abemaciclib in women with disease progression after endocrine therapy.
Indications:
1Hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer.
2HR-positive advanced breast cancer in postmenopausal women with disease progression following endocrine therapy.
3HR-positive, HER2-negative advanced or metastatic breast cancer in postmenopausal women in combination with ribociclib.
Package Insert for Fulvestrant Injection
Please read the instructions carefully and use as directed by your physician
[General Information]
Generic name: fulvestrant injection
Active ingredient: fulvestrant.
Chemical name:
7α-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17β-diol
Chemical structure:
Molecular formula: C32H47F5O3S
Molecular weight: 606.77
Excipients: absolute alcohol, benzyl alcohol, benzyl benzoate, and castor oil
[Appearance]
The solution for injection is a clear, colorless to yellow, viscous liquid.
[Indications]
Fulvestrant is indicated for the treatment of hormone receptor (HR)-positive locally advanced or metastatic breast cancer in postmenopausal women (including natural and surgical menopause) who had disease recurrence on or after adjuvant endocrine therapy or progression following endocrine therapy.
[Strength]
5ml:0.25g
[Usage]
Adults (including the elderly)
The recommended dose is 500 mg at intervals of one month, with an additional 500 mg dose given two weeks after the initial dose.
Paediatric population
Fulvestrant is not recommended for use in children and adolescents as safety and efficacy have not been established in this group of patients.
Special populations
Renal impairment
No dose adjustments are recommended for patients with mild to moderate renal impairment (creatinine clearance ≥30 ml/min). Safety and efficacy have not been evaluated in patients with severe renal impairment (creatinine clearance <30 ml/min), and, therefore, caution is recommended in these patients (see section [Special warnings and precautions for use]).
Hepatic impairment
No dose adjustments are recommended for patients with mild to moderate hepatic impairment. However, as fulvestrant exposure may be increased, fulvestrant should be used with caution in these patients. There are no data in patients with severe hepatic impairment (see sections 4.3, 4.4 and 5.2 [Contraindications], [Special warnings and precautions for use], and [Pharmacokinetics]).
Method of Administration
Fulvestrant injection should be administered as two consecutive 5 ml injections by slow intramuscular injection (1-2 minutes/injection), one in each buttock (gluteal area).
Caution should be taken if injecting fulvestrant injection at the dorsogluteal site due to the proximity of the underlying sciatic nerve.
Instructions for administration
NOTE: Caution should be taken if injecting fulvestrant injection at the dorsogluteal site due to the proximity of the underlying sciatic nerve. (see section [Special warnings and precautions for use]).
Warning - Do not autoclave safety needle (Safety GlideTMNeedle) before use. Hands must remain behind the needle at all times during use and disposal.
For each of the two syringes:
• Remove glass syringe barrel from tray and check that it is not damaged.
• Peel open the safety needle (SafetyGlideTM Needle) outer packaging.
• Parenteral solutions must be inspected visually for particulate matter and discolouration prior to administration.
• Hold the syringe upright on the ribbed part (C).
With the other hand, take hold of the cap (A) and
carefully tilt back and forth (see Figure 1).
• Remove the cap in a straight upward direction.
To maintain sterility do not touch the syringe tip
(see Figure 2).
• Attach the safety needle to the Luer-Lok and twist
until firmly seated (see Figure 3).
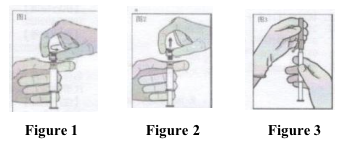
• Check that the needle is locked to the Luer connector before moving out of the vertical plane.
• Pull shield straight off needle to avoid damaging needle point.
• Transport filled syringe to point of administration. Remove needle sheath.
• Expel excess gas from the syringe.
• Administer intramuscularly slowly (1-2 minutes/injection) into the buttock (gluteal area). For user convenience, the needle bevel-up position is oriented to the lever arm (see Figure 4).
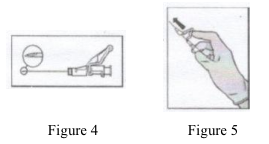
• After injection, immediately apply a single-finger stroke to the activation assisted lever arm to activate the shielding mechanism (see Figure 5).
NOTE: Activate away from self and others. Listen for click and visually confirm needle tip is fully covered.
Disposal
Pre-filled syringes are for single use only.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
[Adverse Reactions]
This section provides information based on all adverse reactions from clinical studies, postmarketing studies or spontaneous reports. In the pooled dataset of fulvestrant monotherapy, the most frequently reported adverse reactions were injection site reactions, asthenia, nausea, and increased hepatic enzymes (ALT, AST, ALP).
The following frequency categories for adverse drug reactions (ADRs) were calculated based on the fulvestrant 500 mg treatment group in pooled safety analyses of studies that compared fulvestrant 500 mg with fulvestrant 250 mg [CONFIRM (Study D6997C00002), FINDER 1 (Study D6997C00004), FINDER 2 (Study D6997C00006), and NEWEST (Study D6997C00003) studies], or from FALCON (Study D699BC00001) alone that compared fulvestrant 500 mg with anastrozole 1 mg. Where frequencies differ between the pooled safety analysis and FALCON, the highest frequency is presented. The frequencies in Table 1 were based on all reported adverse drug reactions, regardless of the investigator assessment of causality.
Table 1. Adverse Drug Reactions Reported in Patients Treated with Fulvestrant 500mg
Frequency | System organ class | Adverse reactions |
Very common (≥10%) | General disorders and administration site conditions; Hepatobiliary disorders; Gastrointestinal disorders; Immune system disorders; Musculoskeletal and connective tissue disorders; Skin and subcutaneous tissue disorders Vascular disorders | Astheniaa, injection site reactionsb
Elevated hepatic enzymes (ALT, AST, ALP) aNausea; Hypersensitivity reactions e Joint and musculoskeletal pain d
Rash e
Hot flushes e |
Common (≥1%- <10%) | Nervous system disorders; Hepatobiliary disorders; Vascular disorders; Gastrointestinal disorders; Metabolism and nutrition disorders; Infections and infestations; Musculoskeletal and connective tissue disorders; Reproductive system and breast disorders; Blood and lymphatic system disorders; General disorders and administration site conditions | Headache; Elevated bilirubin a; Venous thromboembolism a; Vomiting, diarrhea; Anorexia a;
Urinary tract infections Back pain a
Vaginal haemorrhage e
Reduced platelet count e
Neuropathy peripheral e, sciatica e |
Uncommon (≥0.1%- <1%) | Hepatobiliary disorders; Reproductive system and breast disorders; | Hepatic failure c,f, hepatitis f, elevated gamma-GT f Vaginal moniliasis f, leukorrhea f |
General disorders and administration site conditions; Immune system disorders | Injection site haemorrhage f, injection site haematoma f, neuralgia c,f; Anaphylactic reactions |
a: Includes adverse drug reactions for which the exact contribution of fulvestrant cannot be assessed due to the underlying disease.
b: The term injection site reactions does not include the terms injection site haemorrhage, injection site haematoma, sciatica, neuralgia and neuropathy peripheral.
c: The event was not observed in major clinical studies (CONFIRM, FINDER 1, FINDER 2, NEWEST).
The frequency has been calculated using the upper limit of the 95% confidence interval for the point estimate. This is calculated as 3/560 (where 560 is the number of patients in the major clinical studies), which equates to a frequency category of ‘uncommon’.
d: Includes: arthralgia, and less frequently musculoskeletal pain, myalgia and pain in extremity.
e: Frequency category differs between pooled safety dataset and FALCON.
f: ADR was not observed in FALCON.
[Contraindications]
Hypersensitivity to the active substance or to any of the excipients.
Pregnancy and lactation (see [Fertility, Pregnancy and Lactation]).
Severe hepatic impairment (see sections [Special Warnings and Precautions for Use] and [Pharmacokinetics]).
This product contains benzyl alcohol and is contraindicated for intramuscular administration to children.
[Special Warnings and Precautions for Use]
Fulvestrant should be used with caution in patients with mild to moderate hepatic impairment (see sections [Usage], [Contraindications], and [Pharmacokinetics]).
Fulvestrant should be used with caution in patients with severe renal impairment (creatinine clearance less than 30 ml/min).
Due to the intramuscular route of administration, fulvestrant should be used with caution if treating patients with bleeding diatheses, thrombocytopenia or those taking anticoagulant treatment.
Thromboembolic events are commonly observed in women with advanced breast cancer and have been observed in clinical studies with fulvestrant. This should be taken into consideration when prescribing fulvestrant to patients at risk.
Injection site related events including sciatica, neuralgia, neuropathic pain, and peripheral neuropathy have been reported with fulvestrant injection. Caution should be taken while administering fulvestrant at the dorsogluteal injection site due to the proximity of the underlying sciatic nerve (see sections [Usage] and [Adverse Reactions]).
There are no long-term data on the effect of fulvestrant on bone. Due to the mechanism of action of fulvestrant, there is a potential risk of osteoporosis.
Interference with estradiol antibody assays:
Due to the structural similarity of fulvestrant and estradiol, fulvestrant may interfere with antibody based-estradiol assays and may result in falsely increased levels of estradiol.
Use with caution in athletes.
Effects on ability to drive and use machines:
Fulvestrant has no or negligible influence on the ability to drive or use machines. However, since asthenia has been reported very commonly with fulvestrant, caution should be observed by those patients who experience this adverse reaction when driving or operating machinery.
[Fertility, Pregnancy and Lactation]
Women of childbearing potential
Patients of child-bearing potential should use effective contraception during treatment with fulvestrant injection and for 2 years after the last dose.
Pregnancy
Fulvestrant is contraindicated in pregnancy (see section [Contraindications]). Fulvestrant has been shown to cross the placenta after single intramuscular doses in rat and rabbit. Studies in animals have shown reproductive toxicity including an increased incidence of foetal abnormalities and deaths (see section [Pharmacology and Toxicology]). If pregnancy occurs while taking fulvestrant, the patient must be informed of the potential hazard to the foetus and potential risk for loss of pregnancy.
Breast-feeding
Breast-feeding must be discontinued during treatment with fulvestrant. Fulvestrant is excreted in milk in lactating rats. It is not known whether fulvestrant is excreted in human milk. Considering the potential for serious adverse reactions due to fulvestrant in breast-fed infants, use during lactation is contraindicated (see section [Contraindications]).
Fertility
The effects of fulvestrant on fertility in humans has not been studied.
[Paediatric Population]
Fulvestrant is not recommended for use in children and adolescents as safety and efficacy have not been established in this group of patients.
[Geriatric Population]
In the clinical trials 9238IL/0020 and 92381L/0021 for registration in Europe and North America, which included patients aged ≥65 years, the objective response rates were 16% and 11% respectively for patients aged 65 years and over in the fulvestrant treatment group when tumour response was evaluated by age. In the clinical trial D6997L00004 for registration of fulvestrant 250mg in China, which included 35 patients aged ≥65 years, no comparative analysis was performed due to the limited sample size. In the clinical trial D6997100021 for registration of fulvestrant 500mg in China, which included 27 patients aged ≥65 years, no comparative analysis was performed due to the limited sample size.
[Drug-Drug Interactions]
Studies of co-administration of fulvestrant with midazolam, a substrate of CYP3A4, indicate that therapeutic doses of fulvestrant have no inhibitory effects on CYP 3A4.
A clinical study with rifampin, an inducer of CYP 3A4, and a clinical study with ketoconazole, a potent inhibitor of CYP 3A4, showed no effect on the pharmacokinetics of fulvestrant. Therefore, dosage adjustment is not necessary in patients co-prescribed CYP 3A4 inhibitors or inducers.
There are no known drug-drug interactions. Fulvestrant does not significantly inhibit any of the major CYP isoenzymes, including CYP 1A2, 2C9, 2C19, 2D6, and 3A4 in vitro, and studies of co-administration of fulvestrant with midazolam indicate that therapeutic doses of fulvestrant have no inhibitory effects on CYP 3A4 or alter blood levels of drug metabolized by that enzyme. Also, aAlthough fulvestrant is partly metabolized by CYP 3A4, a clinical study with rifampin, an inducer of CYP 3A4, showed no effect on the pharmacokinetics of fulvestrant.
[Overdosage]
There are isolated reports of overdose with fulvestrant injection in humans. If overdose occurs, symptomatic supportive treatment is recommended. Animal studies have shown no effects other than those related directly or indirectly to antiestrogen activity with intramuscular doses of fulvestrant higher than the recommended human dose.
[Pharmacology and Toxicology]
Mechanism of action
Many breast cancers have estrogen receptors (ER), and the growth of these tumors can be stimulated by estrogen. Fulvestrant is an estrogen receptor antagonist that binds to the estrogen receptor in a competitive manner with affinity comparable to that of estradiol. Fulvestrant downregulates the ER protein in human breast cancer cells. Fulvestrant blocks the trophic actions of estrogens without any partial agonist (estrogen-like) activity.
In vitro studies demonstrated that fulvestrant is a reversible inhibitor of the growth of tamoxifen-resistant, as well as estrogen-sensitive human breast cancer (MCF-7) cell lines. In in vivo tumor studies, fulvestrant delayed the establishment of tumors from xenografts of human breast cancer MCF-7 cells in nude mice. Fulvestrant inhibited the growth of established MCF-7 xenografts and of tamoxifen-resistant breast tumor xenografts.
Fulvestrant showed no agonist-type effects in in vivo uterotropic assays in immature or ovariectomized mice and rats. In in vivo studies in immature rats and ovariectomized monkeys, fulvestrant blocked the uterotrophic action of estradiol. In postmenopausal women, the absence of changes in plasma concentrations of FSH and LH in response to fulvestrant treatment (250 mg monthly) suggests no peripheral steroidal effects.
In a clinical study in postmenopausal women with primary breast cancer treated with single doses of fulvestrant 15-22 days prior to surgery, there was evidence of increasing down regulation of ER with increasing dose. This was associated with a dose-related decrease in the expression of the progesterone receptor, an estrogen-regulated protein. These effects on the ER pathway were also associated with a decrease in Ki67 labeling index, a marker of cell proliferation.
Toxicology
Fulvestrant solution for injection and other formulations of fulvestrant were well tolerated in animal species used in multiple dose studies. Local reactions, including myositis and granulomata at the injection site were attributed to the vehicle but the severity of myositis in rabbits increased with fulvestrant, compared to the saline control. In toxicity studies with multiple intramuscular doses of fulvestrant in rats and dogs, the antiestrogenic activity of fulvestrant was responsible for most of the effects seen, particularly in the female reproductive system, but also in other organs sensitive to hormones in both sexes. Arteritis involving a range of different tissues was seen in some dogs after chronic (12 months) dosing.
In dog studies following oral and intravenous administration, effects on the cardiovascular system (slight elevations of the S-T segment of the ECG [oral], and sinus arrest in one dog [intravenous]) were seen. These occurred at exposure levels higher than in patients (Cmax >15 times) and are likely to be of limited significance for human safety at the clinical dose.
Genotoxicity
Fulvestrant was not mutagenic or clastogenic in multiple in vitro tests with and without the addition of a mammalian liver metabolic activation factor (bacterial mutation assay in strains of Salmonella typhimurium and Escherichia coli, in vitro cytogenetics study in human lymphocytes, mammalian cell mutation assay in mouse lymphoma cells and in vivo micronucleus test in rat.
Reproductive toxicity
In female rats, fulvestrant administered at doses ≥0.01 mg/kg/day (0.6% the human recommended dose based on body surface area [BSA in mg/m2]), for 2 weeks prior to and for 1 week following mating, caused a reduction in fertility and embryonic survival. No adverse effects on female fertility and embryonic survival were evident in female animals dosed at 0.001 mg/kg/day (0.06% the human dose based on BSA in mg/m2). Restoration of female fertility to values similar to controls was evident following a 29-day withdrawal period after dosing at 2 mg/kg/day (equivalent to the human dose based on BSA in mg/m2). The effects of fulvestrant on the fertility of female rats appear to be consistent with its antiestrogenic activity. The potential effects of fulvestrant on the fertility of male animals were not studied, but in a 6-month toxicology study, male rats treated with intramuscular doses of 15 mg/kg/30 days, 10 mg/rat/30 days, or 10 mg/rat/15 days fulvestrant showed a loss of spermatozoa from the seminiferous tubules, seminiferous tubular atrophy, and degenerative changes in the epididymides. Changes in the testes and epididymides had not recovered 20 weeks after cessation of dosing. These fulvestrant doses correspond to 1.3-, 1.2-, and 3.5-fold the systemic exposure [AUC0-30 days] achieved in women receiving the recommended dose of 500 mg/month.
Administration of fulvestrant prior to and up to implantation caused embryonic loss at daily doses that were 0.6% of the daily maximum recommended human dose based on mg/m2. Effects on embryo-fetal development consistent with antiestrogenic activity occurred when fulvestrant was administered to rats during organogenesis at doses ≥0.1 mg/kg/day (approximately 6% of the recommended human doses based on mg/m2). Fulvestrant increased the incidence of fetal abnormalities in rats (i.e., tarsal flexure of the hind paw at the dose of 2mg/kg/day, equivalent to the human dose based on mg/m2). Non-ossification of the odontoid and ventral tubercle of the first cervical vertebra at doses ≥ 0.1 mg/kg/day. Fulvestrant caused fetal loss at the dose of 2mg/kg/day. Pregnancy loss also occurred when fulvestrant was administered to pregnant rabbits during organogenesis at the dose of 1 mg/kg/day (equivalent to the human dose on a mg/m2 basis). Further, in rabbits dosed at 0.25 mg/kg/day (30% of the human dose on a mg/m2 basis), increases in placental weight and postimplantation loss were observed. Fulvestrant was associated with an increased incidence of fetal variations in rabbits (backwards displacement of the pelvic girdle, and 27 pre-sacral vertebrae at 0.25 mg/kg/day IM; 30% of the human dose on a mg/m2 basis) when administered during the period of organogenesis.
Fulvestrant is found in rat milk at levels significantly higher (approximately 12-fold) than plasma after administration of 2 mg/kg. Drug exposure in rodent pups from fulvestrant-treated lactating dams was estimated as 10% of the administered dose. In a study in rats of fulvestrant at 10 mg/kg given twice or 15 mg/kg given once (less than the recommended human dose based on mg/m2) during lactation, offspring survival was slightly reduced.
Carcinogenesis
Two-year carcinogenesis studies were conducted in rats and mice. Positive findings were observed in both species. Rats were treated at intramuscular doses of 15 mg/kg/30 days, 10 mg/rat/30 days, and 10 mg/rat/15 days. These doses correspond to 0.9-, 1.5-, and 3-fold (in females) and 0.8-, 0.8-, and 2-fold (in males) the systemic exposure [AUC0-30 days] achieved in women receiving the recommended dose of 500 mg/month. An increased incidence of benign ovarian granulosa cell tumors and testicular Leydig cell tumors was evident, in females dosed at 10 mg/rat/15 days and males dosed at 15 mg/rat/30 days, respectively.
Mice were treated at oral doses of 0, 20, 150, and 500 mg/kg/day. These doses correspond to 0-, 0.8-, 8.4-, and 18-fold (in females) and 0.8-, 7.1-, and 11.9-fold (in males), the systemic exposure (AUC0-30 days) achieved in women receiving the recommended dose of 500 mg/month. There was an increased incidence of sex cord stromal tumors (both benign and malignant) in the ovary of mice at doses of 150 and 500 mg/kg/day. Induction of such tumors is consistent with the pharmacology-related endocrine feedback alterations in gonadotropin levels caused by an antiestrogen.
[Pharmacokinetics]
Absorption
After administration of fulvestrant long-acting intramuscular injection, fulvestrant is slowly absorbed and maximum plasma concentrations (Cmax) are reached after about 5 days. Administration of fulvestrant 500 mg regimen achieves exposure levels at, or close to, steady state within the first month of dosing (mean [CV]: AUC 475 [33.4%] ng.days/ml, Cmax 25.1 [35.3%] ng/ml, Cmin 16.3 [25.9%] ng/ml, respectively). At steady state, fulvestrant plasma concentrations are maintained within a relatively narrow range with up to an approximately 3-fold difference between maximum and trough concentrations. After intramuscular administration, the exposure is approximately dose-proportional in the dose range 50 to 500 mg.
Distribution
Fulvestrant is subject to extensive and rapid distribution. The large apparent volume of distribution at steady state (Vdss) of approximately 3 to 5 l/kg suggests that distribution is largely extravascular. Fulvestrant is highly (99%) bound to plasma proteins. Very low density lipoprotein (VLDL), low density lipoprotein (LDL), and high density lipoprotein (HDL) fractions are the major binding components. No interaction studies were conducted on competitive protein binding. The role of sex hormone-binding globulin (SHBG) has not been determined.
Biotransformation
The metabolism of fulvestrant has not been fully evaluated, but involves combinations of a number of possible biotransformation pathways analogous to those of endogenous steroids. Identified metabolites (includes 17-ketone, sulphone, 3-sulphate, 3- and 17-glucuronide metabolites) are either less active or exhibit similar activity to fulvestrant in anti-estrogen models. Studies using human liver preparations and recombinant human enzymes indicate that CYP3A4 is the only P450 isoenzyme involved in the oxidation of fulvestrant; however, non-P450 routes appear to be more predominant in vivo. In vitro data suggest that fulvestrant does not inhibit CYP450 isoenzymes.
Elimination
Fulvestrant is eliminated mainly in metabolised form. The major route of excretion is via the faeces (about 90%), with less than 1% being excreted in the urine. Fulvestrant has a high clearance, 11±1.7 ml/min/kg, suggesting a high hepatic extraction ratio. The terminal half-life (t1/2) after intramuscular administration is governed by the absorption rate and was estimated to be 50 days.
Special populations
In a population pharmacokinetic analysis of data from phase 3 studies, no difference in fulvestrant’s pharmacokinetic profile was detected with regard to age (range 33 to 89 years), weight (40-127 kg) or race.
Renal impairment
Mild to moderate impairment of renal function did not influence the pharmacokinetics of fulvestrant to any clinically relevant extent.
Hepatic impairment
The pharmacokinetics of fulvestrant has been evaluated in a single-dose clinical study conducted in women with mild to moderate hepatic impairment (Child-Pugh class A and B). A high dose of a shorter duration intramuscular injection formulation was used. There was up to about 2.5-fold increase in AUC in women with hepatic impairment compared to healthy subjects. In patients administered fulvestrant, an increase in exposure of this magnitude is expected to be well tolerated. Women with severe hepatic impairment (Child-Pugh class C) were not evaluated.
Gender
Following administration of a single intravenous dose, there were no pharmacokinetic differences between men and women or between premenopausal and postmenopausal women. Similarly, there were no differences between men and postmenopausal women after intramuscular administration.
Race
In the advanced breast cancer treatment trials, the potential for pharmacokinetic differences due to race have been evaluated in 294 women including 87.4% Caucasian, 7.8% Black, and 4.4% Hispanic. No differences in fulvestrant plasma pharmacokinetics were observed among these groups. In a separate trial, pharmacokinetic data from postmenopausal ethnic Japanese women were similar to those obtained in non-Japanese patients.
[Storage Conditions]
Store and transport refrigerated (2°C - 8°C). Keep the pre-filled syringe in the original package in order to protect from light.
[How Supplied]
Two prefilled syringes
Pre-filled syringe, 2 syringes/pack, with two safety needles for connection to the barrel.
[Shelf Life]
48 months
[Specification]
YBH05492022
[Approval Number]
GYZZ H20223421
[Marketing Authorization Holder]
Name: Hangzhou Jiuyuan Gene Engineering Co., Ltd.
Address: No. 23, No. 8th Street, Hangzhou Economic and Technological Development Zone
[Manufacturer]
Name: Hangzhou Jiuyuan Gene Engineering Co., Ltd.
Address: No. 23, No. 8th Street, Hangzhou Economic and Technological Development Zone
Post Code: 310018
Tel: 86-0571-86910099 ext.
Fax: 86-0571-86911688
Website: www.china-gene.com